22 Jan Design Simulation Modeling – Reduce MedTech Project Risk and Accelerate Time to Market
Managing technical and schedule risk is one of the toughest challenges during development of a complex medical device. Historically, the predictability of these programs has been set back by the “surprise” result of an empirical test after much of the design work is completed. The “surprise” can result in questioning of the initial assumptions and understanding of a cutting-edge technology, sending the design team back to the drawing board and impacting the overall program schedule.
This blog post summarizes Key Tech’s approach to intelligent design simulation, for use on the toughest product development challenges.
First-Principals and Focus
Simulation is a valuable tool during the development of a complex medical device. We focus on understanding and incorporating first-order principals of physics and engineering into our simulation models, providing a strong foundation onto which nuanced second-order features of the system can be added with confidence. We identify the parameters that are expected to vary and impact system performance and structure the model to allow design simulation modeling across the full range of those input parameters. In many cases, “perfect is the enemy of good” and we are careful to limit the scope of the simulation to only capture the critical aspects of the system and reduce MedTech product risk. Our goal is to answer the fundamental questions of how a given system works across a range of conditions, but with sufficient detail to capture the subtle corner-cases that can derail a project late in the game.
Drawing on the expertise and knowledge of the Key Tech design team, once the model is assembled, we confirm that the results match our intuition and observations and data available in literature. We then verify the design simulation model behavior with empirical tests. This combination of quantitative and qualitative verification results in a robust model that performs well across the full range of conditions.
The Power of Simulation.
Our design simulation modeling provides insight into the sensitivity of a system to input variables (e.g. part tolerances, environmental conditions, process parameters, etc.) early in the design process. This insight informs many aspects of the system architecture including tolerance definition, sensor selection and location, control system design, and critical subsystem specifications. Understanding of the system sensitivities enables the team to intelligently define requirements to meet the intent of the product, while not overly burdening the design team during system verification; allowing the team to accelerate time to market.
Later in the development cycle, modeling multiple physical prototypes for variations of a given design can be complex, time-consuming and expensive, so we leverage the models to focus our testing, including informing the scope of Design of Experiments (DOEs) and eventual verification testing. Design simulation models provide a means to run virtual tests with meaningful output, quickly evolving the design to de-risk and meet performance, reliability and cost objectives.
Keeping it Simple!
Design simulation modeling enables medical device design engineers to probe areas of a system that are difficult to access during design and testing, but which may be critical to the function of the overall system. For example, fluid temperature in a microfluidic channel is very difficult to measure without impacting the system, but intelligent simulation can enable MedTech designers to model the fluid temperature based on the temperature of surfaces that are easily accessible during testing. This tool enables us to design simple tests, monitoring easy-to-measure variables, while leveraging the design simulation model to provide insight into those difficult-to-measure areas buried deep in the system. The use of design simulation reduces workload and time to complete elaborate design verification tests and positions a program to move to design validation testing on-time with high confidence.
Does it Work?
Key Tech has successfully applied its design simulation modeling expertise to many engineering challenges over the years, including those described below.
- Patient thermoregulation model – We modeled the human body’s thermoregulation system to inform the design and performance requirements of the CoolStat, a device that cools patients for a range of indications. The model was based on a wide range of literature sources and was baselined against actual patient data under a range of conditions. See an example here.
- Pump accuracy model – We developed a detailed model to understand the variables that impact volumetric accuracy of peristaltic pumps in the device. The model informed early device architecture, detailed design, and eventual DOE and verification test plans. See an example here.
- Measurement uncertainty model – Highly complex ultrasonic uncertainty models were developed to establish tolerance limits on critical variables, including hardware dimensions, temperature sensor performance, and electronics and firmware timing. The early models provided a foundation that guided development and testing throughout the program. See examples here and here.
- DNA process thermal model – Detailed thermal transient finite element analyses (FEA), often coupled with computational fluid dynamics (CFD) models, have been developed for multiple IVD projects to guide the development of high-performance thermal systems. These thermal systems often need to meet strict assay requirements (e.g. thermal cycling to enable rapid PCR ) and the models allow rapid system optimization, enable intelligent component selection, and provide context for early discussions with the assay team about the tradeoffs between assay performance and instrument cost and complexity. See examples here and here.
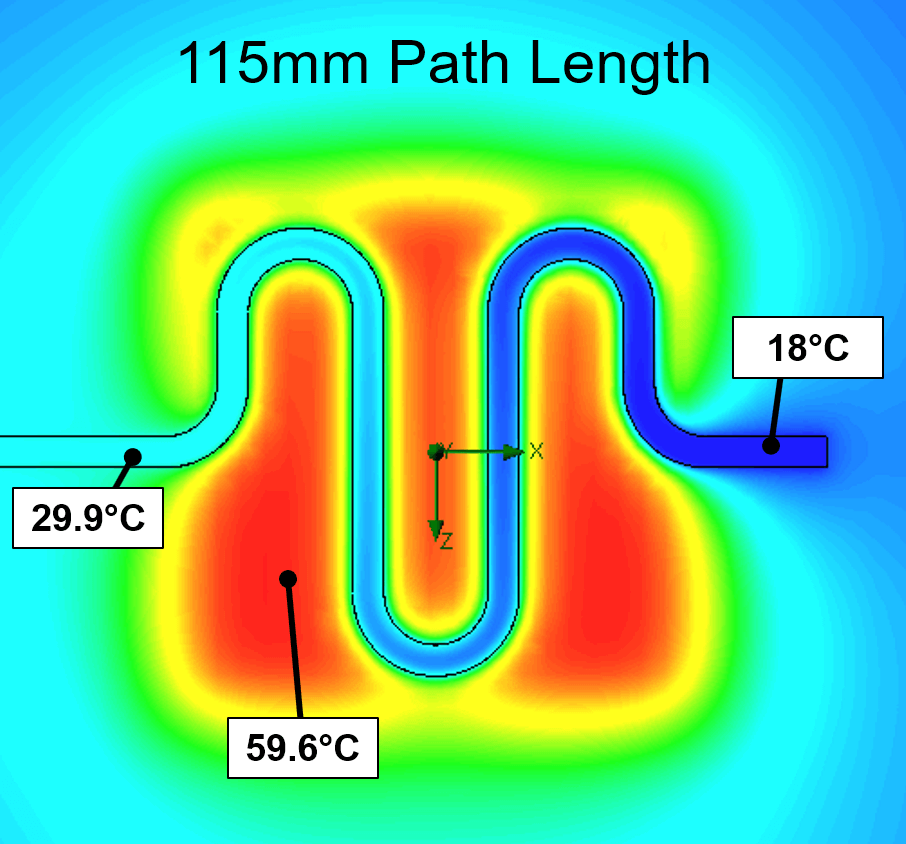
- Micro-fluidic model – We have modeled many microfluidic systems over the years to better understand and optimize mixing dynamics in microfluidic channels and chambers or to predict sensitivity of liquid flow rate to variations in driving pressure and channel dimensions. These analyses drive the design of microfluidic cartridges and the systems that interface with them.
- Dynamic model with optimized impact dynamics – We modeled the dynamics of a system over a period of several microseconds after impact with a fixed object, with the goal of understanding the micron scale vibration of the components of the system. The small displacements and short time scale of the impact made it impossible to capture with a high-speed camera. Modeling was critical to developing an understanding of the behavior of the system.
A Stable Employee-Owned Team, with a Rich History Designing Medical Devices
Design simulation is not just a popular term or catchphrase. Here at Key Tech we use it frequently because we know it optimizes medical device development. The more our highly experienced team uses design simulation modeling, the more our clients experience an efficient, reliable and de-risked design; reducing “surprises” in the verification and validation process. Ultimately, our clients gain accelerated time to market with efficient, ISO 13485 compliant development processes.
Key Tech can help! As an employee-owned company, every person at Key Tech is invested in the success of our clients and their projects. Whether during development or providing an evaluation of on-market products, you can feel secure in Key Tech’s engineering expertise and commitment. Please TalkToUs to see how we can support your product development imperatives.
- Design Simulation Modeling – Reduce MedTech Project Risk and Accelerate Time to Market - January 22, 2020
- Product Design: How to Choose the Right Sensor - September 9, 2015
- Face to Face - November 6, 2013


